Key points
Liquids and gases are both fluidSubstances that can flow and change shape easily, like water. Liquids and gases are both examples of fluids.. Fluids can flow or be poured, because the particles in them are able to move around freely and can change positions.
Liquids and gases exert pressureThe amount of force acting on a certain area. Pressure is measured in pascals (Pa) which are the same as newtons per metre squared (N/m²). on any objects immersed in them and on surfaces in contact with them, for example the walls of their containers. This is because the particles in a fluid are free to move around and can therefore expand and ‘bounce’ off surfaces.
The movement and space between particles in a fluid affects the amount of pressure the fluid exerts. This means that the pressure in a fluid changes depending on its height, depth and temperature.
Pressure in gases
The particles in a gas move quickly in random directions. Therefore the particles regularly bump into each other and the walls of their container.
These collisions exert pressure on the walls of the container and on any objects surrounded by the gas.
If the temperature of a gas is increased, the particles move faster, so they hit the walls of the container more often. This causes the pressure to increase.
Decreasing the volumeThe amount of space that something occupies. The amount of space within closed surfaces. of the container also increases the pressure exerted by the gas. This is because the rate at which the particles collide with the surfaces increases because there are more particles in a smaller space.
Try this experiment at home to find out about gas pressure.

Image caption, Partially inflate a balloon, so that it is slightly larger than a tennis ball, like the one in the picture.
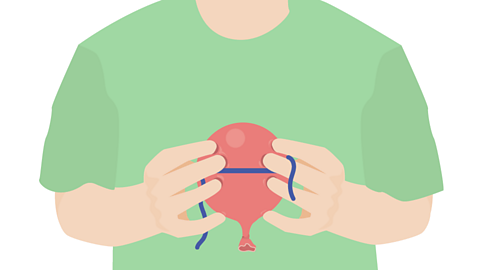
Image caption, Measure the circumference of the balloon (all the way around the middle) using a piece of string.
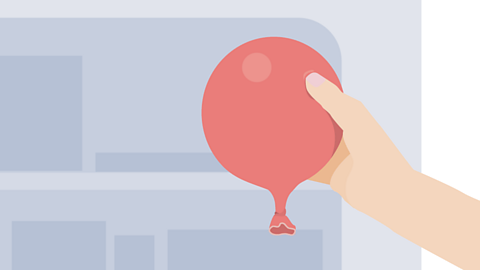
Image caption, Place the balloon in a freezer compartment for at least an hour.
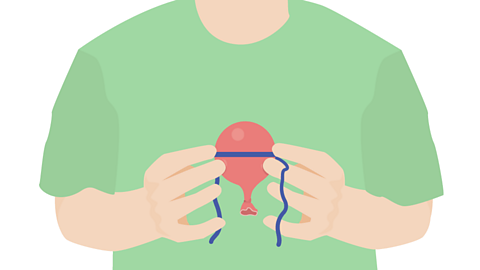
Image caption, Measure the circumference of the balloon again. You should find it is smaller than before.
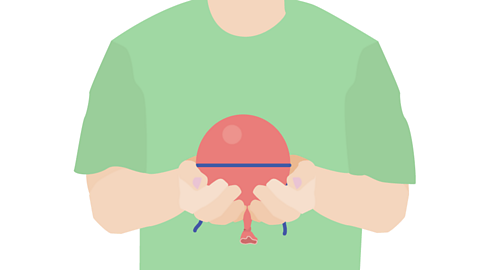
Image caption, Leave your balloon somewhere at room temperature for an hour and then measure its circumference again. You should find it has returned to its original size.
1 of 5
Why did the balloon in the freezer shrink?
When a gas is cooled, the particles have less kinetic (movement) energy and get closer together, this means that they take up less room. This also means that the pressure is lower. This is why the balloon in the freezer shrank.
Atmospheric pressure
Earth's atmosphereAn atmosphere is the relatively thin layers of gases surrounding a planet or other celestial body is a mixture of gases, and is comprised mainly of nitrogen and oxygen. The nitrogen and oxygen particles in the atmosphere are constantly colliding with us and exert a pressure of around 100,000 pascals (Pa)The units used to measure pressure, represented using the letters Pa. A pressure of one pascal is the same as a force of 1 newton spread over a surface area of 1 square metre. at sea level.

Imagine someone climbing Mount Everest. As the climber moves up through the atmosphere, the pressure decreases. This is because particles in the atmosphere are attracted to Earth's gravityGravity is an attraction force that pulls things toward one another. This size of the gravitational attraction is related to the mass of an object. , so there are fewer particles higher up.
At higher altitudes the weight of the air particles above the climber is less and the air is thinner, meaning the particles in the air are further apart. This means that the particles collide with the climber less frequently.
By the time the climber reaches the highest point of Mount Everest, the air pressure is only around 33,700 Pa approximately one third of the atmospheric pressure at sea level.
What is altitude sickness?
Altitude sickness is a set of symptoms caused by rapidly ascending to areas high above sea level. It can cause headaches, make you feel sick and short of breath.
This happens because there are fewer air particles at high altitudes and this includes fewer oxygen molecules. Altitude sickness is a reaction the body has to quickly reducing oxygen levels. People that live at high altitudes do not suffer from altitude sickness as their body is accustomed to the pressure and oxygen levels.
Pressure in liquids
Just like gases, liquids exert pressure on objects due to collisions between the liquid particles and the object.
The amount of pressure exerted depends on both the densityAll substances are made of particles. Density is a measure of how close together particles are. Closely packed particles have a higher density than particles that are spread out. of the liquid and the depth of the liquid. The deeper you go:
- the greater the weight of liquid above the object
- the greater the liquid pressure
The Mariana Trench in the western Pacific is the deepest part of the ocean and is nearly 11 km below sea level. The pressure at that depth is estimated to be around 1.1 × 10⁸ Pa (110,000,000 Pa).

Try this
Try this experiment at home to find out about pressure in liquids.
You will need the following items:
- Large plastic bottle with lid
- 1 small pin
- A pencil
- Sticky tape
- Water
- A large container (sink/bath)
Watch the video to see how to do this experiment yourself

Image caption, Click to see a step-by-step slideshow.

Image caption, YOU WILL NEED - An empty plastic bottle, some sticky tape, a pin, a pencil and a jug of water.

Image caption, 1. Take a large plastic bottle. Using a pin make 3 small holes at different heights of the bottle (ask an adult to help you). Use the pencil to widen the holes as shown in the video.

Image caption, 2. Cover the holes with sticky tape.

Image caption, 3. Fill the bottle with water. Make sure the water is above the 3 holes and place the lid on it.

Image caption, 4. Next carry your filled bottle carefully to your container (holding the bottle horizontally will stop the water escaping from the holes). Stand the bottle upright and remove the lid then quickly remove the tape firstly from the top hole, then the middle hole and finally the bottom hole.

Image caption, 5. Watch the streams of water escaping from each of the holes. You should find that the water from the lowest hole is ejected at the highest speed and travels furthest. This is because the water above it creates the highest pressure at the bottom of the bottle. The Arrows show how the water pressure forces the water out of the bottle.
1 of 7
Why do soda drinks fizz when you open them?
The drink is made fizzy by having dissolved carbon dioxide in the liquid when it is under pressure in the closed bottle.When you open the bottle, you release the pressure and the liquid cannot hold all of the carbon dioxide at lower pressure, so the gas quickly bubbles to the top, causing your drink to fizz.
Pressure and buoyancy
An object in a liquid experiences a force called upthrustA force caused by pressure in liquids. A liquid pushing against something can cause objects to float if the upthrust equals the weight.. This is due to the particles in the liquid colliding with the surface of the object, which exerts pressure.
An object placed in a liquid will begin to sink. As it sinks, the liquid pressure on it increases and so the upthrust increases.
For a floating object, the upthrust is equal and opposite to the object’s weight. An object will continue to sink if its weight is greater than the maximum upthrust.
Test your knowledge
GCSE exam dates 2025
Find out everything you need to know about the 2025 GCSE exams including dates, timetables and changes to exams to get your revision in shape.

More on Solids, liquids and gases
Find out more by working through a topic
- count4 of 4

- count1 of 4

- count2 of 4
