What are biological molecules?
The food we eat – our diet – is made up of different biological molecules which give us energy and contain chemicals we need to grow and repair and help cells function in our body.
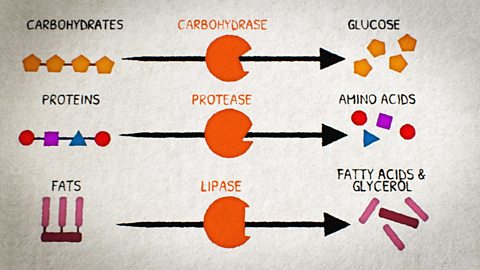
Carbohydrates
Carbohydrates provide energy. There are two types - simple and complex.
Simple carbohydrates are sugars, like glucose and lactose, and are found in food like biscuits or energy bars.

Complex carbohydrates are:
Cellulose – found in plant cell walls providing support to the cell
Starch – an energy store in plants. Broken down by amylase into glucose, for respiration
Glycogen – an energy store in animals. Can be broken down into glucose when needed
Complex carbohydrates can be found in foods including whole-grain products, such as bread, rice and pasta, some fruits, legumes, and starchy vegetables, such as sweet potatoes.
Fats

Fats are an energy store, providing twice as much energy as carbohydrates and proteins.
They are made up of fatty acids and glycerol.
Foods high in fat include cheese, butter and oils.

What are proteins made up of?

Proteins are made up of long chains of amino acids and are essential for growth and repair of cells.
They serve as:
structural molecules eg in muscles, skin, nails and
functional molecules eg enzymes, hormones, antibodies
Foods high in protein include fish and eggs.
Reagents and food testing
Different tests can detect carbohydrates, proteins, and lipids in food.
These tests use a chemical (reagent) that changes colour if certain molecules are present.
Sometimes, the food needs to be crushed or mixed with water before adding the reagent.
| Food sample | Reagent | Method | Initial colour | Colour of positive result |
|---|---|---|---|---|
| Reducing sugar | Benedict's | Add Benedict’s solution to the food and heat in a water bath | Blue | Brick red precipitate |
| Starch | Iodine solution | Add iodine solution | Yellow-brown | Blue-black |
| Protein/amino acids | Biuret | Add Biuret reagent | Blue | Lilac/purple |
| Fat | Ethanol | Add ethanol to the food, then add water | Colourless | White emulsion |
Practical - How to investigate the energy content of food
A simple investigation can be conducted to investigate the energy content of a food sample.
Procedure
- Add 20cm³ of water to a boiling tube in a retort stand.
- Record the starting temperature.
- Ignite 1g of food on a mounted needle.
- Hold the burning food under the boiling tube until fully burned (relight if needed).
- Record the final water temperature.
- Record results in a table.
- Repeat steps 1-6 with different foods.
Results:
| Food | At start (°C) | At end | Difference |
|---|---|---|---|
| Corn crisps | 20 | 42 | 22 |
| Potato crisps | 20 | 36 | 16 |
Analysis: Use the formula to calculate energy released. This formula will be provided in the exam.
1cm³ of water = 1g
Energy (J) = mass of water (g) x temp rise (°C) x 4.2.
Corn crisps = 20g x 22 x 4.2 = 1848J
= 1.8kJ
How much energy was in the potato crisps? Answer = 20g x 16 x 4.2 = 1344J
= 1.3kJ
Conclusion:
The corn crisps had more energy because they had a larger increase in temperature.
Sources of error:
The results obtained from this experiment are usually lower than the actual energy content of the food because during the experiment, the entire food sample may not be burned, some energy is lost to the air and some is used to heat the glass of the boiling tube.
Improvements:
- reduce heat loss to surroundings by using a calorimeterA machine used to measure the energy involved in a chemical process.
- use a temperature sensorA device that measures temperature and converts it to an electrical signal. Temperature sensors are used in many applications, including air conditioning, home appliances, and industrial systems
- stir water: stirring improves accuracy because it ensures even heat distribution by preventing temperature variations within the water. Without stirring, hot and cold spots can form, leading to inconsistent readings depending on where the thermometer is placed. By stirring, the heat is evenly distributed, ensuring that the temperature measurement is more reliable and representative of the whole test-tube.
Independent variable: type of food
Dependent variable: temperature / °C
Controlled variables: mass of food, mass of water, distance of burning food from water
Test your knowledge
Watch: Biological Molecules
More on Living processes
Find out more by working through a topic
- count3 of 6
- count5 of 6
- count6 of 6