Key points
- Chemical reactions make new chemicals.
- Atoms are rearranged during a chemical reaction, but the number of atoms does not change.
- Evidence of chemical reactions includes a large temperature change, bubbles, or a colour change.
- Chemical reactions can be represented using equations.
Video
Watch this video to find out what happens to atoms in a chemical reaction.
Chemical reactions.
Compounds are chemical substances that contain more than one element. They're created during a chemical reaction where atoms are rearranged into new compound molecules. For example, if carbon atoms react with oxygen atoms they form carbon dioxide molecules. Carbon dioxide is present in the atmosphere and contributes to global warming.
We write this reaction as a word equation and as the chemical formula, CO₂.
This tells us there are two oxygen atoms for every single carbon atom.
Have you ever baked a cake? Do you think it would be easy to get the ingredients back after cooking? Explain your reasoning in terms of a chemical reaction.

It would be impossible to get the ingredients back from the cooked cake. This is because a chemical reaction has taken place between ingredients such as flour, baking powder, sugar, eggs and butter.
Evidence for chemical reactions

Evidence for a chemical reaction can include any of the following:
- Bubbles – Many chemical reactions you see in the science lab make a chemical which is a gas, so you see bubbles.
- A colour change – If the new chemicals are a different colour from the original chemicals, there will be a colour change.
- A large energy change – Many chemical reactions give off lots of energy, like burning, and a few absorb energy, so they feel cold.


A new substance must be produced for a chemical reaction to take place.
The water inside a kettle has bubbles but that’s just because it is boiling. Bubbles of steam form in boiling water. These bubbles do not indicate a new substance has been formed because, when cooled, the steam condenses back into liquid water. Boiling is a physical changeA reversible process which does not involve chemical bonds being broken or made. No new substances are made., not a chemical reaction.
There is a colour change when you add a teabag to a mug of boiling water. Why is this not a chemical reaction?
The chemicals in the tea are dissolving in the water, and dissolving is not a chemical reaction – it is a physical change.
How are chemical reactions used?

Lots of chemical reactions are used to make useful new chemicals, like plastics and medicines.
Many useful chemical reactions involve burning fuels to release energy. These reactions heat our homes, power our cars and generate lots of the electricity that we use.
Cooking uses chemical reactions to make foods which are safe and tasty to eat.

Video
Watch this video about how a chef uses chemical reactionWhen chemical bonds are broken and made between atoms, so that new substances (compounds or elements) are made. in their kitchen to make honeycomb.
A case study video on how a chef applies the concept of reactions to their job
Every day in my kitchen there’s reactions happening all around me all the time, from me coming in lighting the stove, baking, cooking.
We’re making honeycomb, which the kids absolutely love and we do this in the kids cookery classes.
First of all we need to add the honey…
Next we are going to add the glucose but I am actually going to use some golden syrup.
Some sugar. A little bit of water.
And finally we add the bicarbonate of soda and this is where the magic happens.
And you get this chemical reaction and it looks like lava.
So we’re gonna leave that there for about an hour and then once it hardens we smash it into pieces and it’s ready to eat.
What happened when the chef added the bicarbonate of soda to the hot liquid in their pan?
There were lots of bubbles.
Making CO₂ from vinegar and baking soda
If you mix vinegar and baking soda, a reaction will occur, where lots of bubbles of carbon dioxide gas form.
You can do this reaction at home but make sure you don’t get any of the chemicals in your eyes.Ethanoic acid is the chemical name for vinegar.Sodium bicarbonate is also known as baking soda.

Image caption, WHAT YOU NEED: vinegar, bicarbonate of soda, a small glass, a teaspoon and a candle.

Image caption, STEP 1: Light the candle. REMEMBER: lit candles are a fire risk, so make sure you have an adult with you!

Image caption, STEP 2: Pour 100 millilitres of vinegar into a large glass. The vinegar contains ethanoic acid.

Image caption, STEP 3: Add a small teaspoon of bicarbonate of soda. The scientific name for this is sodium bicarbonate. The mixture should start to foam as the sodium bicarbonate reacts with the ethanoic acid and produces bubbles of carbon dioxide gas.

Image caption, STEP 4: Pour the carbon dioxide onto the flame. The CO₂ gas is heavier than air so pours down onto the burning candle wick. It displaces the oxygen fuelling the flame and puts it out!
1 of 5
A particle model of a chemical reaction
In this animation, watch how sodium bicarbonate reacts with ethanoic acid.
A model of sodium bicarbonate and ethanoic acid reacting
Notice that…
- there are the same number of particles at the beginning of the reaction as there are after it
- the two reactants have broken down and reformed as three products
- sodium bicarbonate + ethanoic acid → sodium ethanoate + water + carbon dioxide
Can you recall the formula for water and carbon dioxide?
The formula for water is H₂O.The formula for carbon dioxide is CO₂.
Chemical reactions and physical changes

- Physical changes, such as melting, boiling and dissolving, do not make new chemicals. They are usually easy to reverse.
- In a chemical reaction, chemical bonds between atoms are broken and made, so the atoms get rearranged into new substances.
- The simplest kind of chemical reactions involve two elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table. reacting together to make a compoundA pure substance made from two or more elements which are chemically bonded in a fixed ratio. .

Which two elements react together to make the compound sulfur dioxide?
Sulfur and oxygen.
Representing a chemical reaction
See what happens in the chemical reaction when iron and sulfur are heated. A sulfide is a chemical compound containing the element sulfur.

Image caption, The test tube is partly filled with a mixture of iron and sulfur.
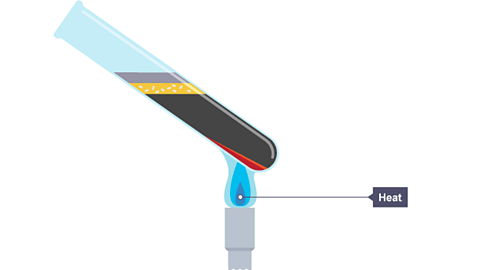
Image caption, The mixture is heated strongly using a bunsen burner.
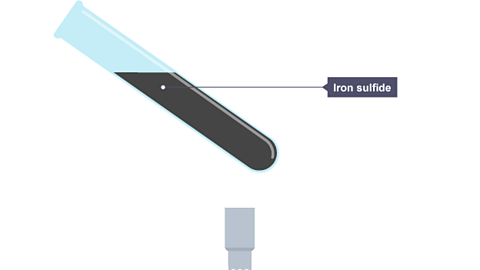
Image caption, The test tube now contains iron sulfide.
1 of 3
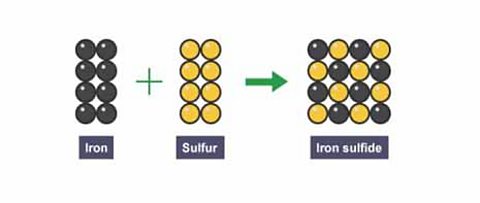
What has happened to the total number of atoms during the chemical reaction to form iron sulfide?
It has not changed. There are 16 atoms before the reaction and 16 atoms after the reaction.
Working scientifically
Types of data
If you carry out an investigation you will record evidence of any changes. The evidence you record is called data and it may be quantitative or qualitative.
Quantitative data
Quantitative data can be counted, measured, and expressed using numbers.
Quantitative data can be discrete (counted) or continuous (measured).
For example, if a reaction releases a lot of energy we can use a thermometer and measure the temperature rise in degrees Celsius ©.
Qualitative data (words)
Qualitative data describes qualities or characteristics which are often observable.
For example, “the colour changed from white to blue”.
You must always state what the colour was and what it changed too.

Data recorded in scientific experiments can be quantitative or qualitative.
Find out more about different types of data in science.
Test your knowledge
Teaching resources
Looking for more resources for your chemistry lessons? In this video from the Chemistry Bites series, science presenter Jon Chase explains the Collision theory and four different ways to increase the rate of a reaction.
BBC Teach has thousands of free, curriculum-linked resources to help deliver lessons - all arranged by subject and age group.
GCSE exam dates 2025
Find out everything you need to know about the 2025 GCSE exams including dates, timetables and changes to exams to get your revision in shape.

More on Chemical reactions
Find out more by working through a topic
- count2 of 12

- count3 of 12

- count4 of 12

- count5 of 12
