What are the key learning points?
Each elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table. An element cannot be broken down into anything simpler by chemical means. is represented by a chemical symbol. These symbols can be joined together with numbers to make chemical formulae.
Chemical formulae can be worked out using a ‘swap and drop’ method that uses valencyA measurement of the number of bonds an atom/ion is able to form. It represents the number of electrons lost or gained. numbers for different atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons. and ionElectrically charged particle, formed when an atom gains or loses electrons..
Chemical equations show how reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. interact to make products in a chemical reaction. All chemical symbol equations must be balanced.
There are two other types of chemical equation: ionic equationA chemical equation that shows how positively charged ions join with negatively charged ions to make a compound. and half equationAn equation, involving ions and electrons, that describes the process happening at an electrode. (higher tier only).
What are symbols and formulae?
Chemical symbols
Each element in the periodic table is represented by its own chemical symbol.
A chemical symbol has one or two letters, and it must always start with a capital letter.
Any second letter must always be lower case.
For example, Mg is the correct symbol for magnesium, but mg, mG and MG are wrong.
Take care to write chemical symbols correctly.
Diatomic elements
The formulae for most elements is just their symbol.
The exceptions are some non-metal elements known as diatomic elements .
They contain two atoms of the same element covalent bondA covalent bond is formed by a shared pair of electrons. together.
| Diatomic element | Molecular formula |
|---|---|
| hydrogen | H2 |
| nitrogen | N2 |
| oxygen | O2 |
| fluorine | F2 |
| chlorine | Cl2 |
| bromine | Br2 |
| iodine | I2 |
Key fact
You will need to memorise the seven diatomic elements.
A mnemonic that can help is “Now I Only Have Bright and Clever Friends”.
How to interpret formulae
A formula gives information about the type and the number of each atomThe smallest particle of an element. We often think of atoms as tiny spheres, but in fact they are made from smaller particles called protons, neutrons and electrons. present in a compoundA substance formed when two or more elements are chemically combined..
The formula for sodium sulfate is Na2SO4. It tells you that sodium sulfate contains:
two sodium atoms
one sulfur atom and
four oxygen atoms
The subscript numbers refer to the element before them.
Key fact
If there is just one atom in the formula a ‘1’ is not written.
Question
How many calcium, carbon and oxygen atoms are there in calcium carbonate, CaCO3?
Answer
- 1 calcium atom
- 1 carbon atom
- 3 oxygen atoms
What happens when a formula includes brackets?
Sometimes a formula includes brackets.
The atoms inside the brackets are multiplied by the number outside the brackets.
Aluminium sulfate’s formula is Al2(SO4)3.
It contains:
- 2 atoms of aluminium
- 3 atoms of sulfur
- 12 atoms of oxygen
Question
How many calcium, nitrogen and oxygen atoms are there in calcium nitrate, Ca(NO3)2?
Answer
1 calcium atom
2 nitrogen atoms
6 oxygen atoms
What formulae are used for compounds?
A compound is two or more elements that are chemically combined.
Some compounds are regarded as ‘common’ and their formulae need to be memorised, while for other compounds you will need to work out the formulae yourself.
What are common compounds?
The table below shows some common compounds.
| Compound | Formula | Compound | Formula |
|---|---|---|---|
| Water | H2O | Ammonia | NH3 |
| Carbon monoxide | CO | Hydrochloric acid | HCl |
| Carbon dioxide | CO2 | Nitric acid | HNO3 |
| Sulfur dioxide | SO2 | Sulfuric acid | H2SO4 |
| Nitrogen monoxide | NO | Sodium hydroxide | NaOH |
| Nitrogen dioxide | NO2 | Potassium hydroxide | KOH |
Key fact
You will need to memorise this list of common compounds and their formulae.
How to work out the formula of an ionic compound
It is possible to work out the formula of an ionic compound from its name.
Ionic compounds contain positive and negative ionElectrically charged particle, formed when an atom gains or loses electrons..
You need to be able to work out how many of each ion are required for the formula.
Valency numbers are important for working out formulae.
The valency of an ion tells you how many bonds it is able to form or how many electrons it will lose or gain.
There is a pattern that links the group number of an element to its valency.
| Group number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8/0 |
| Valency | 1 | 2 | 3 | 4 | 3 | 2 | 1 | 0 |
The ‘swap and drop’ method is useful for working out formulae:
Write down the symbols of both elements in the compound.
Look up the group number of the elements and write the valency number to the top right of each element.
Swap the two valency numbers over, and drop them down to the bottom of each symbol.
Write the finished formula.
Example: Magnesium chloride
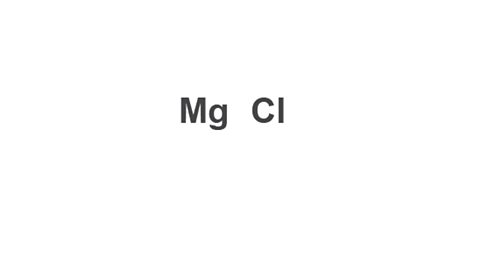
Image caption, 1. Write down the symbols of both elements in the compound.
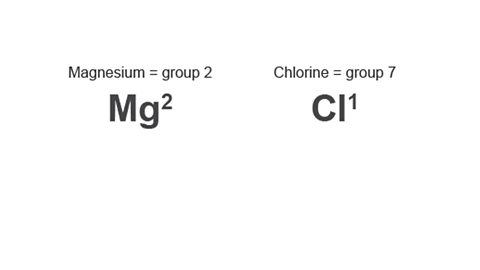
Image caption, 2. Look up the group number of the elements and write the valency number to the top right of each element.
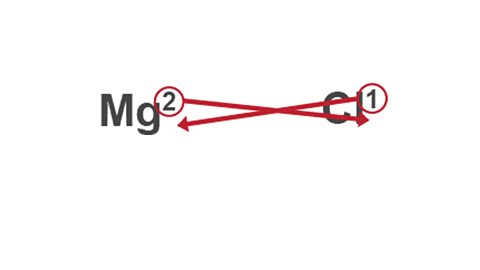
Image caption, 3. Swap the two valency numbers over, and drop them down to the bottom of each symbol.
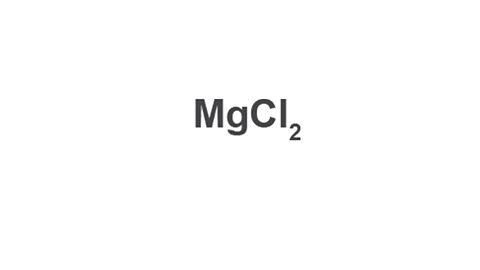
Image caption, 4. Write the finished formula.
1 of 4
Key fact
If both valency numbers are the same, then the numbers cancel out and there is just one of each element in the compound.
Example: Calcium oxide
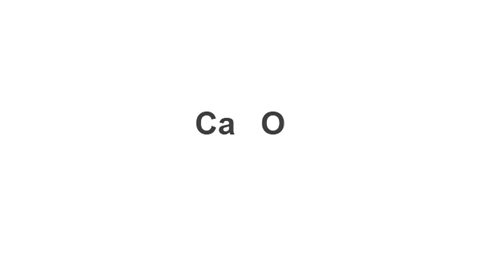
Image caption, 1. Write down the symbols of both elements in the compound.
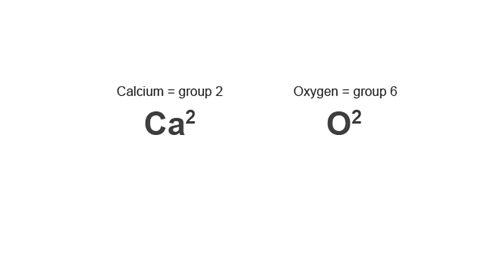
Image caption, 2. Look up the group number of the elements and write the valency number to the top right of each element.
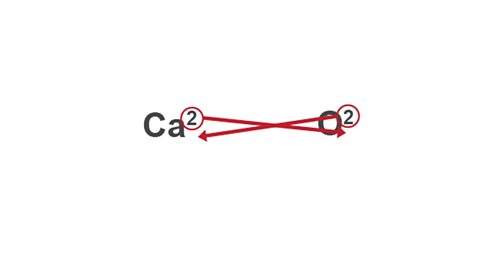
Image caption, 3. Swap the two valency numbers over, and drop them down to the bottom of each symbol.

Image caption, 4. Write the finished formula (both valencies were 2, so they cancel out).
1 of 4
How to work out the formula of a compound containing molecular ions
A molecular ion is a charged particle containing more than one element.
The rules for carrying out a swap and drop in a compound containing a molecular ion are the same as for single elements.
The valency of the molecular ion is simply the size of the charge on the ion.
Example: Sodium hydroxide
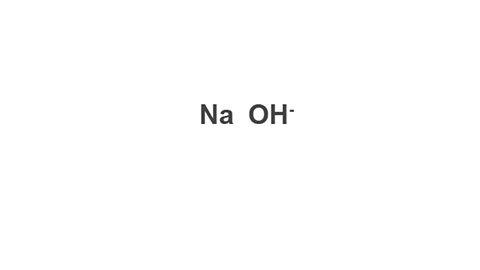
Image caption, 1. Write down the symbol/formula of both parts of the compound.
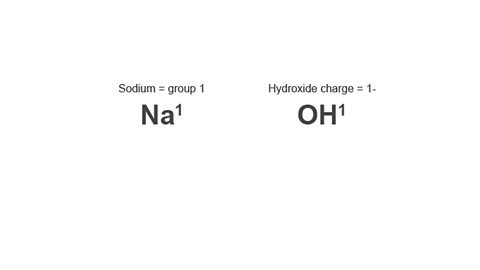
Image caption, 2. The valency of sodium comes from its group number. The valency of hydroxide comes from the number in front of its charge.
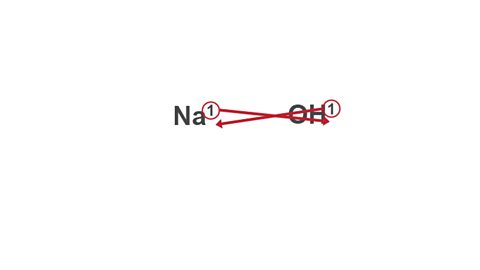
Image caption, 3. Swap the two valency numbers over, and drop them down to the bottom of each symbol.
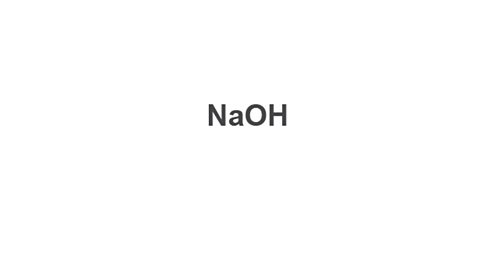
Image caption, 4. Write the finished formula (both valencies were 1, so they cancel out).
1 of 4
In cases where a number greater than one is swapped across to a molecular ionA charged particle containing more than one element., brackets are required to go around the formula of the molecular ion.
Example: Aluminium sulfate
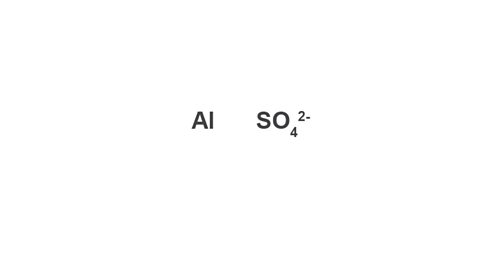
Image caption, 1. Write down the symbol/formula of both parts of the compound. The formula for sulfate is on the back of the Data Leaflet.

Image caption, 2. The valency of aluminium comes from its group number. The valency of sulfate comes from the number in front of its charge.

Image caption, 3. Swap the two valency numbers over, and drop them down to the bottom of each symbol.

Image caption, 4. Write the finished formula (as sulfate has a number greater than 1 beside it, brackets are required).
1 of 4
What are equations?
In a chemical reaction, the substances that react together are called reactants and the substances that are made are called products.
Word equations
All reactions can be represented in word equations, which must contain an arrow (→)
reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. → productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (→).
For example, magnesium can react with oxygen to produce magnesium oxide:
Magnesium + oxygen → magnesium oxide
Key fact
Chemical equations contain an arrow and not an equals sign.
The arrow means 'reacts to make'.
Question
Nitrogen and hydrogen react together to form ammonia. What is the word equation for the reaction?
Answer
nitrogen + hydrogen → ammonia
What are balanced symbol equations?
No atoms are created or destroyed in a chemical reaction – they are just rearranged.
Balanced symbol equations show the original arrangements and the new ones.
What are the key rules about balancing symbol equations?
Each element must have equal numbers of atoms on each side of the equation.
You are not allowed to change any formulae to balance an equation (e.g. you cannot change MgO into MgO2).
You can add a ‘big’ number in front of a formula to multiply all the atoms in that formula by that number.
A method that can help with balancing equations is the ‘bubble method.’
Click through the arrows below to see how this method works.
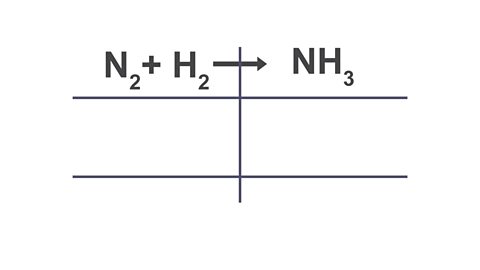
Image caption, 1. Write out the equation that needs to be balanced and draw a table through the equation.
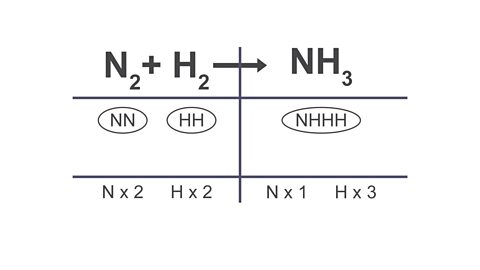
Image caption, 2. In the table, list out the atoms that are in each formula and group them using bubbles. Use this to count the atoms on each side of the equation.
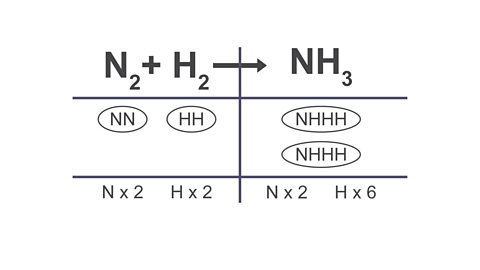
Image caption, 3. If you need to add extra atoms of an element on one side, add an extra bubble that contains the atoms you need.
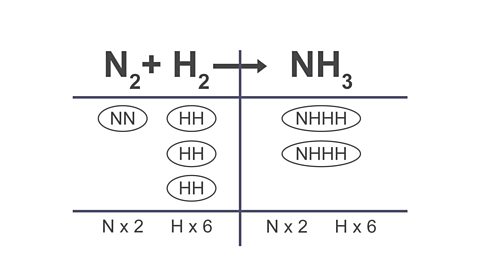
Image caption, 4. Add extra bubbles until the equation is balanced
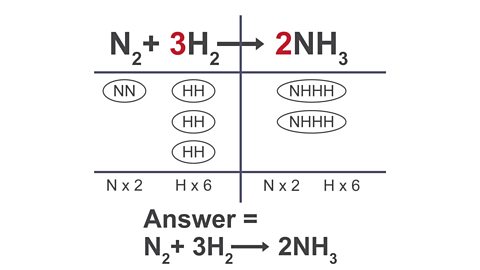
Image caption, 5. Count the number of bubbles for each formula and write this as a large number in front of each formula
1 of 5
Question
Balance the following equation:
Al + O2 → Al2O3
Answer
4Al + 3O2 → 2Al2O3
What are state symbols?
Chemical equations use state symbols:
| State symbol | Meaning |
|---|---|
| (s) | solid |
| (l) | liquid |
| (g) | gas |
| (aq) | aqueous solution (dissolved in water) |
Question
An iron nail is placed in a solution of blue copper(II) sulfate.
Over time, a brown solid appears and the solution becomes colourless.
This is because copper and a solution of iron(II) sulfate forms.
Add state symbols to the balanced chemical equation for the reaction of iron with copper(II) sulfate.
Fe + CuSO4 → FeSO4 + Cu
Answer
Fe(s) + CuSO4(aq) → FeSO4(aq)+ Cu(s)
What are ionic equations? (Higher tier only)
Ionic compounds react when they are dissolved.
However, not all the ions react – some spectator ionAn ion that is present in a reaction but does not take part. Its formula will be the same on both sides of the equation. do not react and remain unchanged.
Spectator ions are not shown in an ionic equation.
There are two different types of ionic equations:
- Neutralisation
- Displacement
What is neutralisation?
When an acid reacts with an alkali, the acid’s hydrogen ionElectrically charged particle, formed when an atom gains or loses electrons. react with the alkali’s hydroxide ions to produce water.
The other ions are left unchanged.
For example, when hydrochloric acid (HCl) is mixed with potassium hydroxide (KOH), the hydrogen ions (H+) react with the hydroxide ions (OH-).
The chloride (Cl-) and potassium (K+) ions are spectator ions.
Ionic equation for neutralisation, including state symbols: H+(aq) + OH-(aq) → H2O(l)
The ionic equation for neutralisation is always the same for any neutralisation reaction.
It is worth learning it off by heart.
What is displacement?
In displacement reactions, the more reactive metal displaces (takes the place of) a less reactive metal from a metal compound.
For example, when magnesium (Mg) reacts with copper(II) sulfate (CuSO4), the magnesium atoms react with the copper(II) ions in the copper(II) sulfate.
The sulfate ions are spectator ions and do not take part in the reaction.
Final ionic equation, including state symbols: Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
Question
Chlorine reacts with potassium bromide to make potassium chloride and bromine:
Cl2 + 2KBr → 2KCl + Br2
Write the ionic equation for this reaction.
Answer
Cl2 + 2Br- → 2Cl- + Br2
What are half equations? (Higher tier only)
A half equation contains only a single element.
It shows the change of an atom/molecule into an ion, or vice versa.
The process for writing a balanced ionic equation is as follows:
- Write down the reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. and the productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (→). .
- Balance the atoms.
- Write the total charge underneath each species in the equation.
- Balance the charge by adding electrons.
Worked example
Write the half equation for the conversion of chlorine molecules into chloride ions.
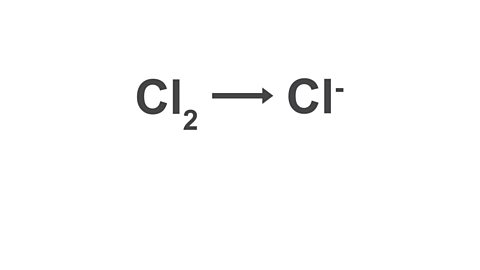
Image caption, 1. Write down the reactant and the product.
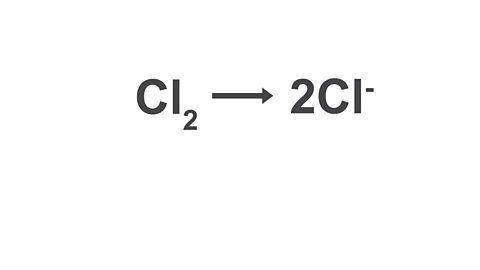
Image caption, 2. Balance the atoms.
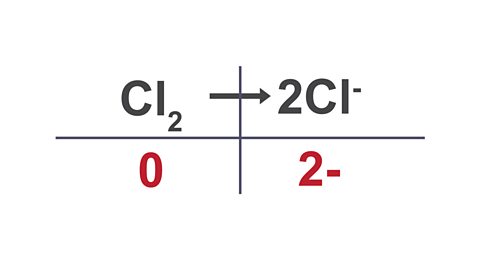
Image caption, 3. Write the total charge underneath each substance in the equation.
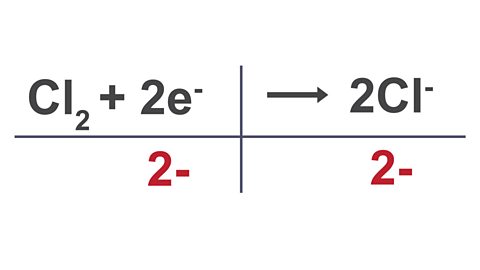
Image caption, 4. Balance the charge by adding electrons (remember electrons have a negative charge).
1 of 4
Answer = Cl2 + 2e- → 2Cl-
Question
Write a balanced half equation for the conversion of oxide ions into oxygen molecules.
Answer = 2O2- → O2 + 4e-
How much do you know about symbols, formulae and equations?
More on Unit 1: Structures, trends, chemical reactions, quantitative chemistry and analysis
Find out more by working through a topic
- count6 of 10

- count7 of 10

- count8 of 10

- count9 of 10
