Past papers and mark schemes
Here you can find past papers for OCR 21st Century GCSE Chemistry. Use the links below to download question papers and mark schemes. If you’re unsure whether you need Foundation or Higher papers, consult your teacher.
June 2023
Breadth in chemistry - Foundation tier - Past paper | Mark scheme
Breadth in chemistry - Higher tier - Past paper | Mark scheme
Depth in chemistry - Foundation tier - Past paper | Mark scheme
Depth in chemistry - Higher tier - Past paper | Mark scheme
June 2022
Breadth in chemistry - Foundation tier - Past paper | Mark scheme
Breadth in chemistry - Higher tier - Past paper | Mark scheme
Depth in chemistry - Foundation tier - Past paper | Mark scheme
Depth in chemistry - Higher tier - Past paper | Mark scheme
November 2021
Breadth in chemistry - Foundation tier - Past paper | Mark scheme
Breadth in chemistry - Higher tier - Past paper | Mark scheme
Depth in chemistry - Foundation tier - Past paper | Mark scheme
Depth in chemistry - Higher tier - Past paper | Mark scheme
These resources were created to support your exam preparation. The past papers and mark schemes here belong to OCR and are shared with their permission.
Practicing questions
Practising questions will help you to prepare for your chemistry exam. By working through example problems you can build your confidence and sharpen your understanding of key topics.
These worked examples not only show you how to approach problems step-by-step but also include tailored tips for each topic to help you develop the skills to tackle similar questions on your own.
Use these questions to identify areas you feel confident in and those that need more practice.
Remember, the questions on the exam paper are not released until the day of the exam. The examples on this page are a guide to the topics and question structures that could be on the paper.
Top tips: Rates of reaction
1. Practicals and experiments
- Past paper trend: Rates of reactions questions are often linked to experiments.
- Exam tip: This is often investigating the effect of changing temperature on the reaction of sodium thiosulfate and hydrochloric acid.
Practise writing the method for this practical:
- independent variable: temperature (the variable that is changed).
- dependent variable: time taken for a cross to disappear as the solution becomes cloudy (the variable that is measured).
- controlled variable: volume of the reactants (the variable that is kept constant).
2. Drawing graphs
- Past paper trend: Questions often require drawing a graph from a table of data and describing or explaining it.
- Exam tip: Take care when drawing the graph and use a pencil to allow for corrections if needed. Make sure each point is accurately plotted. When instructed to draw a line of best fit it should be a straight line or smooth curve passing through as many points as possible.
Check if the question asks for a description of the graph. If it does, explain what the data trend shows. Using the above graph as an example:
As the temperature increases so does the rate of reaction. This is not linear. As the temperature increases the rate of reaction increases, making the line steeper at higher temperatures.
In some cases the question may ask for an explanation of the graph. This involves providing reasons for the shape of the graph and is often easiest using the word ‘because’. For example:
At higher temperatures the molecules possess more kinetic energy and so are moving more quickly. This means there are more successful collisions, so the rate of reaction is faster.
3. Calculating the mean rate of a reaction
- Past paper trend: Graph questions often ask you to calculate the mean rate of a reaction for a certain length of time or the rate at a specific point.
- Exam tip: To calculate the mean rate of reaction for the first five minutes (for example) locate the time point on the horizontal axis (x-axis). Draw a vertical line up to the line of best fit then a horizontal line across to the vertical axis (y-axis). Divide the value on the y-axis by the value on the x-axis to find the rate.
To determine the rate at a specific point draw a large tangent to the curve at that point. The rate is the gradient of the tangent at this point. Divide the change in the y-axis by the corresponding change in the x-axis.
The steepness of the red tangent indicates the reaction is fastest at the start. The less steep green tangent and the shallow blue tangent show the reaction slows down over time.
Worked example question
Here is an example of the type of question you may encounter in the exam and an example answer with an explanation of how the marks have been awarded.
Practise the process of writing a short plan for all six-mark questions.
Spending one minute planning and five minutes writing often scores more highly than just jumping in and writing for six minutes. Try to make as many key points as there are marks on offer. For this six-mark question try to write a minimum of six points.
Question
Describe the method you would use to investigate the rate of a reaction. Include the independent variable, dependent variable and one controlled variable in your answer. [6 marks]
Answer:
Add 50 cm3 of sodium thiosulfate solution to a conical flask. Place this onto a piece of paper with a cross drawn onto it. Add 10 cm3 of dilute hydrochloric acid to the conical flask and swirl it. Start a stopwatch. Record the time taken for the cross to disappear. Repeat the experiment at different temperatures. The independent variable is the temperature. The dependent variable is the time taken for the cross to disappear. A controlled variable is the volume of the reactants.
Explanation of marks:
1 mark for the reactants (sodium thiosulfate and hydrochloric acid)
1 mark for piece of paper with a cross drawn onto it
1 mark for stopping timing when the cross disappears
1 mark for the independent variable (temperature)
1 mark for the dependent variable (time taken for the cross to disappear)
1 mark for a controlled variable (volume or concentration of reactants)
This question tests multiple skills including remembering the method and identifying the variables, justifying the 6 marks.
Top tips: Ionic and covalent bonding
1. Drawing atoms
- Past paper trend: Questions often ask you to draw atoms involved in ionic and covalent bonding.
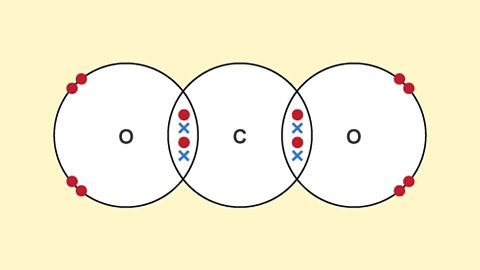
- Exam tip: The question is expecting a dot-and-cross diagram. Remember to use dots for one atom and crosses for the other to show which electrons have been transferred. Draw square brackets around the ions and include their charges outside the top right corner.
Covalent bonding involves the sharing of electrons between atoms. As with the ionic bond example draw a dot-and-cross diagram, taking care to show the shared electrons. There are no square brackets and no charges in covalent bonding.
2. Linking structures to their properties
Past paper trend: Questions often involve linking the structure of ionic and covalent molecules to their properties.
Exam tip: Properties of substances include their:
- melting and boiling points
- state of matter at room temperature
- electrical conductivity
Example answer: ionic compounds
Ionic compounds are made from giant repeating arrangements of positive and negative ions forming a three-dimensional lattice. The positively and negatively charged ions are attracted to each other by strong electrostatic forces of attraction. There are usually a large number of these in a lattice and so a lot of energy is required to overcome these. Ionic compounds have high melting and boiling points and so are solids at room temperature. Ionic lattices do not possess spare electrons and so ionic compounds can only conduct electricity if they have melted or dissolved in water.
Example answer: covalent molecules
Covalent bonding can form small molecules such as water and carbon dioxide. There are strong covalent bonds between the atoms but only weak intermolecular forces between the different molecules. These forces are more easily overcome, so most small covalent molecules have low melting and boiling points. These are liquids or gases at room temperature. Small covalent molecules have no overall charge and so cannot conduct electricity even when liquid or dissolved in water.
Example answer: covalent structures
Covalent bonding can also form giant structures like diamond and graphite. These have strong covalent bonds between all atoms. These are not easily overcome so giant covalent molecules have high melting and boiling points. They are therefore solids at room temperatures. Most giant covalent structures have no charged particles and so cannot conduct electricity. Graphite is an exception.
3. Comparing structures
- Past paper trend: A common question about covalent bonding compares the structure and electrical conductivity of diamond and graphite.
- Exam tip: It is easy to start the answer with ‘Each carbon atom in…’ and then state the number of bonds:
Each carbon atom in diamond has four covalent bonds. This means there are no spare electrons and so diamond cannot conduct electricity. Each carbon atom in graphite has only three covalent bonds. This means each has one spare electron. These flow from one atom to the next and so graphite can conduct electricity.
Worked example question
Diamond is a giant covalent structure. Sodium chloride is an ionic substance. Explain how the structure of these substances determines their properties. [6 marks]
Answer:
The carbon atoms in diamond all possess four strong covalent bonds. This means that there are no spare electrons to carry charge and so diamond is not an electrical conductor. A lot of energy is required to break these bonds, so diamond has a high melting and boiling point. Sodium chloride forms a giant lattice with strong electrostatic forces of attraction between oppositely charged ions. A lot of energy is required to break these bonds, so sodium chloride has a high melting and boiling point. There are no spare electrons to flow in solid sodium chloride and so it is not an electrical conductor. Ionic substances can conduct electricity when molten or dissolved because their ions can flow.
Explanation of marks:
1 mark for each carbon atom in diamond having four strong covalent bonds and no spare electrons
1 mark for explaining that diamond cannot conduct electricity as a result of this
1 mark for lots of energy required to break covalent bonds
1 mark for explaining that diamond has a high melting and boiling point as a result of this
1 mark for strong electrostatic forces of attraction between oppositely charged ions
1 mark for lots of energy required to break electrostatic forces of attraction
1 mark for explaining that sodium chloride has a high melting and boiling point as a result of this
1 mark for explaining that ionic substances can conduct electricity when molten or dissolved because their ions can flow
This question tests multiple skills including describing the structures of these substances and using these to explain their properties, which is why 6 marks are allocated.
Revise chemistry
Explore more chemistry resources with the full range of help from Bitesize.
Bonding, structure and properties podcasts
Learn about bonding, structure and properties of matter for your GCSE chemistry exam, with Dr Sunayana Bhargava and Tulela Pea.

GCSE chemistry - quick-fire quizzes
Foundation and higher exam quizzes based on GCSE chemistry past papers to boost your revision.

Chemistry (single science)
Pinpoint areas to revise from the full list of GCSE chemistry topics.
Support - exams and revision
Exams can be stressful, but don't worry: we've got you. Check out our handy tips and advice for keeping on top of your studies and revision.

More on Exam practice
Find out more by working through a topic
- count1 of 4
- count2 of 4

- count3 of 4
