Key points
- The modern periodic table arranges elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table. into a unique arrangement that can be used for reference.
- Metals are found on the left of the periodic table and non-metals on the right.
- The periodic table is arranged in rows called periods and columns called groups, which can be used to locate any element.
Periodic table activity
Play this game to learn about lots of different elements in the periodic table.
Can water be found in the periodic table?
No.
Water is a compound, H₂O. The periodic table is made up of elements.
Find out more about elements in this learning guide.

Video
Watch this video to find out more information on the periodic tableA table which lists all of the chemical elements and arranges them in a way that is useful. It allows us to spot patterns and make predictions about other elements..
While you're watching, look out for where different elements are found in the periodic table.
Cal: Let's talk about some of the elements on the periodic table, shall we?
Mrs Roberts: So, here I have an empty pop can. This is made of aluminium. Aluminium is an element. Now, each element has a chemical symbol made of one or two letters.
Cal: Right, OK. And what is the chemical symbol for aluminium?
Mrs Roberts: So, the chemical symbol is Al. See if you can see it in the periodic table.
Cal: OK. It is. It's on the right hand side. It's right there. Magic.
OK, well I might have been able to find that, but I'm still a little bit lost on what all of this actually means. So how has the periodic table been put together?
Mrs Roberts: Well, the modern periodic table shows us all of the elements arranged in rows, which are called periods. It has changed a lot over the years as we've learned more and more about atoms. The way we arrange them is in the order of increasing atomic number.
Cal: And what about these vertical columns? I mean, what does all of that mean?
Mrs Roberts: Well, elements that have similar properties to each other are put into vertical columns called groups. Now, the table is called the periodic table because the elements with similar properties occur at regular intervals.
Cal: Ah, OK. Now that does make a bit more sense.
What are the vertical columns of elements in the periodic table called?
The vertical columns in the periodic table are called groups.
The periodic table
In the modern periodic table:
- The elements are arranged in order of increasing atomic number.
- The horizontal rows are called periodElements in a period are in the same horizontal row going across in the periodic table. .
- The vertical columns are called groupElements in a group are in the same vertical column going down in the periodic table. They have similar properties..
- Elements in the same group are similar to each other.
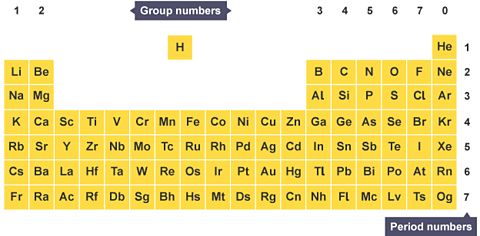
Each square in the table contains an element symbol of one or two letters, not the element name. This makes it less cluttered and easier to find the elements.
- The element symbol always starts with a capital letter.
- Any letters that follow will be lower case letters, for example, helium is He.
Some element symbols are simple. For example, oxygen is O.Others aren’t as straightforward. For example, the element symbol for iron is Fe, from the latin word ferrum for iron.
The arrangement of the periodic table
On the periodic table, metals are found on the left and in the middle, and non-metals are on the right. A zig-zag line divides them.

In most periodic tables hydrogen (H) is shown separately but is still classified as a non-metal. Hydrogen is placed on its own as it is quite a unique element.

Did you know?
Hydrogen has lots in common with the metals in group 1, however, hydrogen is not a metal.

Groups and periods
The periodic table can be read across from left to the right along the rows horizontally. It can also be read up and down the vertical columns.
- Rows of elements are called periods. They go across the whole periodic table from left to right, even if there is a gap. For example, the third period contains sodium (Na) to argon (Ar) elements.
- Columns of elements are called groups and are numbered. For example, group 4 contains carbon © elements through to flerovium (Fl).
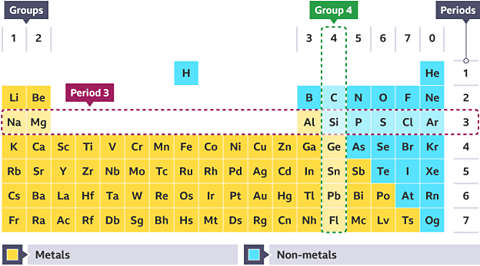
Groups contain elements with similar chemical propertyThe way an element or compound reacts with other chemical substances. and physical propertyA property of an element or compound which can be directly observed or measured. For example, melting point, electrical conductivity, appearance at room temperature. . For example, the metals in group 1 are all reactive, whereas the elements in group 0 are all unreactive gases.
Periodic tables sometimes look different from each other. They can have different labels on the groups or numbers in the rectangles. However, the elements never move places.
The periodic table's layout means we can make predictions about elements based on their position on the periodic table. It is this structure that makes it very useful to scientists.

All element symbols start with a capital letter, but when a symbol has 2 letters in it the second letter is always lower case. For example, the element symbol of magnesium is Mg, not MG.
Working scientifically
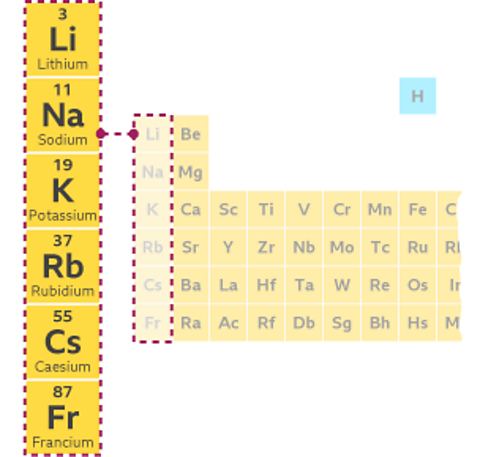
The periodic table arrangement allows us to look for patterns in elements' chemical and physical properties and predict how other elements in the same area might behave.
This is easiest to do in the groups of elements arranged in the vertical columns. Elements in a group have similar physical and chemical properties and show trends.

The table below shows the melting points of most elements in group 1. Melting points are a physical property of an element. The first Group 1 element, lithium, has the highest melting point at 180°C. The melting points then get gradually lower, with the element caesium four places down having a melting point of 28°C.
| Element | Melting point |
|---|---|
| Lithium, Li | 180°C |
| Sodium, Na | 98°C |
| Potassium, K | 63°C |
| Rubidium, Rb | 39°C |
| Caesium, Cs | 28°C |

Did you know?
A hypothesis is a suggestion or prediction made on the basis of some evidence.
The melting points of the elements above francium can be used to make a hypothesisA hypothesis is a suggestion that is made based on some evidence. A hypothesis is then usually tested by gathering more evidence..

Francium is the next element down in group 1. Can you think of a hypothesis for the melting point of francium based on this periodic trend?
We predict that francium would have a lower melting point than caesium.
This hypothesis could be tested by measuring the melting point of francium, although the metal is very hard to isolate and get an accurate figure. However, the range of temperatures measured by scientists is lower than 28°C, so our hypothesis is correct!
Find out more about using a hypothesis in science.
Test your knowledge
Quiz
Teaching resources
Are you a chemistry teacher looking for more resources for your class? Share this short film with your students, in which science presenter Jon Chase uses universal indicator to measure the pH of a solution.
BBC Teach has thousands of free, curriculum-linked resources to help deliver lessons - all arranged by subject and age group.
GCSE exam dates 2025
Find out everything you need to know about the 2025 GCSE exams including dates, timetables and changes to exams to get your revision in shape.

More on Periodic table
Find out more by working through a topic
- count4 of 6

- count5 of 6

- count6 of 6

- count1 of 6
