Key points
- Thermal means heat. Decomposing is the process of breaking down.
- Thermal decomposition is a chemical reaction that happens when a compound breaks down when heated.
Game - thermal decomposition
Play an Atomic Labs experiment exploring the thermal decomposition of different metal carbonates.
You can also play the full game
Baking powder and self-raising flour contain the chemical sodium hydrogencarbonate. This chemical breaks down when heated. What is released that makes cakes rise?
When sodium hydrogencarbonate is heated in the oven, it breaks down and releases carbon dioxide gas, making the cake rise.
Video - thermal decomposition
Watch this video to find out about thermal decomposition reactions.
Look out for the number of reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. there are in a thermal decomposition reaction.
Thermal decomposition reactions
Gethin: Today we're going to be looking at chemical reactions and specifically thermal decomposition. So, Miss Armit, when and where do thermal decomposition reactions happen?
Miss Armit: Well, thermal decomposition occurs at very high temperatures. If a compound is heated it will break down to two or more substances and these can be elements or compounds. So, if we have calcium carbonate and we heat it, it breaks down to produce calcium oxide and carbon dioxide.
Gethin: OK, I know you're poised with your pen and your flip chart. But, before that let's have a look at the top things you need to know about thermal decomposition.
VOICEOVER: Thermal decomposition.
Thermal decomposition reactions usually occur at very high temperatures. In these reactions one compound is heated, for example, copper carbonate and it breaks down to produce two or more substances. In this case, copper oxide and carbon dioxide. The word equation for this reaction is written like this: Copper carbonate produces copper oxide, plus carbon dioxide. Copper carbonate is called the reactant. The chemical substance present at the start of the reaction. Whilst copper oxide and carbon dioxide are the product. The chemical substances which are made as a result of the chemical reaction. Thermal decomposition reactions are common in everyday life.
For example, sodium hydrogen carbonate, also called baking soda, thermally decomposes when you bake muffins. The gas produced in this reaction helps the muffin mixture rise.
Gethin: So, when and how does thermal decomposition occur?
Miss Armit: Well, thermal decomposition occurs at very high temperatures. If a compound is heated it will break down into two or more substances and this happens all the time. Every day, for instance, when you're baking cakes or making honeycomb.
Gethin: So, like, if I am baking a muffin? Essentially, that's a chemical reaction?
Miss Ahmet: Yeah, because what happens when you're making muffins is the same again. The baking soda or the sodium hydrogen carbonate decomposes release this carbon dioxide, which makes the muffins.
Gethin: Yes, OK. So, what other examples are there of thermal decomposition?
Miss Armit: Well, we can start here.
So, here we have got copper carbonate. What do you think it's going to break down into?
Gethin: Copper… Copper… Copper oxide! And carbon dioxide.
Miss Armit: Perfect. So we have got copper oxide and carbon dioxide. What do you think this one here's called?
Gethin: So, that's calcium… Calcium… Calcium carbonate!
Miss Armit: Perfect. Calcium carbonate needs to be heated up to eight hundred twenty degrees to start its decomposition. What you think it will break down into, then?
Gethin: So many questions. Calcium oxide and then carbon dioxide.
Miss Armit: Same again. So, we have got calcium oxide and carbon dioxide.
Another thing to remember is that thermal decomposition is an example of an endothermic reaction. That means the reaction gains energy from all the surroundings and this is why you must constantly be supplied to keep the reaction going.
What are the products when sodium hydrogencarbonate thermally decomposes?
Sodium carbonate, water and carbon dioxide.
What is thermal decomposition?
Thermal means heat. Decomposing is the process of breaking down. Thermal decomposition is a chemical reactionWhen chemical bonds are broken and made between atoms, so that new substances (compounds or elements) are made. that happens when a compound breaks down when heated.
Thermal decomposition reactions happen at high temperatures. The reactants absorb lots of energy as they break down into the productA chemical which is made in a chemical reaction. Products are written on the right of a chemical equation, after the arrow (→). .
The starting compoundA pure substance made from two or more elements which are chemically bonded in a fixed ratio. is the reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. . It breaks down to simpler substances, which could be elementA pure substance which is made from only one type of atom. Elements are listed on the periodic table. or they could be compounds.
Thermal decomposition reactions are examples of endothermicA physical change or chemical reaction that absorbs energy from the surroundings. reactions, and are useful when cooking and baking cakes.
Working scientifically
Working safely with thermal reactions
Cooking involves chemical reactions and often takes place at high temperatures. But you don’t usually see cooks and chefs wearing eye protection.
In the science lab, however, you always wear eye protection when working with chemical reactions.

- In the science lab, eye protection is worn because of the larger amounts of chemicals, harmful chemicals and unfamiliar equipment surrounded by other people carrying out the same investigations.
- Wearing eye protection helps reduce the risk of an accident involving laboratory hazards and reminds us of the need to work safely.
Equations for thermal decomposition
In a thermal decomposition reaction, there is only one reactant, but two or more products.
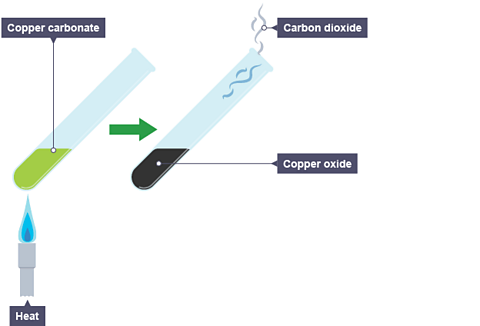
For example, when copper carbonate is heated, it breaks down to produce copper oxide and carbon dioxide.
Copper carbonate is green and copper oxide is black. You can see a colour change from green to black during the reaction. The carbon dioxide produced can be detected using limewater, which turns from clear to cloudy.
The word equation for this reaction is:
copper carbonate → copper oxide + carbon dioxide
The symbol equation for this reaction is:
CuCO₃(s) → CuO(s) + CO₂(g)
Not all thermal decomposition reactions produce a colour change. When magnesium carbonate is heated, it breaks down to produce magnesium oxide and carbon dioxide. There is no change in colour because magnesium carbonate and magnesium oxide are white solids.
The word equation for this reaction is:
magnesium carbonate → magnesium oxide + carbon dioxide
The symbol equation for this reaction is:
MgCO₃(s) → MgO(s) + CO₂(g)

A state symbol is a symbol used in chemical equations to show if a substance is a solid (s), a liquid (l), a gas (g), or an aqueous solution (aq).
Read more about state symbols and word equations.
What is produced when calcium carbonate is heated?
Calcium oxide and carbon dioxide.
Test your knowledge
GCSE exam dates 2025
Find out everything you need to know about the 2025 GCSE exams including dates, timetables and changes to exams to get your revision in shape.

More on Chemical reactions
Find out more by working through a topic
- count10 of 12
- count11 of 12

- count12 of 12

- count1 of 12

