What are the main learning points about practical C3?
saltA compound formed during a neutralisation reaction when some or all of the hydrogen ions in an acid are replaced with metal ions or ammonium ions. can be prepared from the reactions of acidCorrosive substance which has a pH lower than 7. Acidity is caused by a high concentration of hydrogen ions..
A pure salt can be prepared from the reaction of an acid with an excess amount of an insolubleAn insoluble substance does not dissolve in water. For example, wax will not dissolve in water. solid, eg copper(II) carbonate.
A pure salt can also be prepared from the reaction of an acid with an exact amount of alkaliA base which is soluble in water. A base is a substance which neutralises an acid. solution.
What is the purpose of prescribed practical C3?
Salts are produced in the reactions of acids.
Some salts can be useful, and so chemists sometimes carry out preparations of pure salts.
There are two different approaches to making a pure salt which are shown in the two experiments below.
The most important part of preparing a salt is that it is pure – there can be no leftover reactantThe chemical present at the start of a reaction. Reactants appear on the left of a chemical equation, before the arrow →. that would make the salt impure.
Experiment 1 – How to make a salt from an insoluble solid and an acid.
In this experiment you will be making a saltA compound formed during a neutralisation reaction when some or all of the hydrogen ions in an acid are replaced with metal ions or ammonium ions. using a metal carbonate.
For example, the salt copper(II) sulfate can be prepared from the reaction of copper(II) carbonate with sulfuric acid:
copper(II) carbonate + sulfuric acid → copper(II) sulfate + water + carbon dioxide
To ensure there is no leftover sulfuric acid in the copper(II) sulfate, an excess of copper(II) carbonate is used to make sure the sulfuric acid is fully neutralised.
The leftover copper(II) oxide solid can be filtered from the solution, leaving the copper(II) sulfate and water behind.
The water can be removed by evaporation, leaving behind pure copper(II) sulfate.
WATCH: Experiment 1
Experiment: Making a soluble salt from a base
What apparatus and chemicals are used in experiment 1?

100 cm3 beakers (x2), glass rod
Tripod, gauze, Bunsen burner, heat proof mat
Filter funnel, filter paper and conical flask
Evaporating basin
Copper(II) carbonate (approx 4 g)
Sulfuric acid (1 mol/dm3 25 cm3)
Follow the safety advice of your teacher.
What are the steps involved in carrying out experiment 1?
Place 4 g of copper(II) carbonate in a beaker.
Warm 25 cm3 of 1 mol/dm3 sulfuric acid in another beaker, the acid should be no hotter than 60оC.
Add the copper(II) carbonate to the sulfuric acid and stir until there is no further reaction and unreacted copper(II) carbonate is present.
Allow to cool and filter the mixture and retain the filtrate.
Heat the filtrate in an evaporating basin on a tripod and gauze until the volume is about one half of what it was originally.
Allow the basin to cool and the crystals will form.
When the crystals have formed, filter if necessary and dry (between two sheets of filter paper or using a desiccator or place in a low temperature oven).
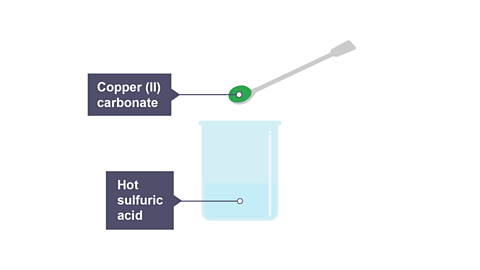
Image caption, 1. Copper(II) carbonate is added to hot sulfuric acid and stirred.
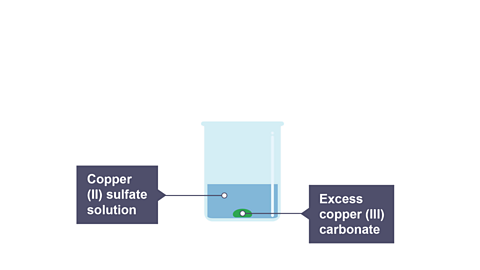
Image caption, 2. Copper(II) sulfate is produced, and the leftover copper(II) carbonate settled to the bottom of the beaker.
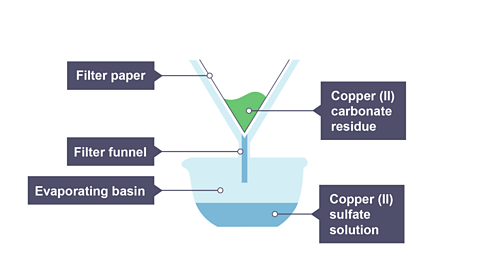
Image caption, 3. The leftover copper(II) carbonate is filtered off, leaving copper(II) sulfate and water in the evaporating dish.
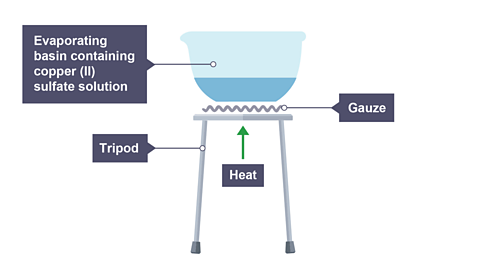
Image caption, 4. Water is removed by evaporation, leaving behind pure copper(II) sulfate solid
1 of 4
Experiment 2: How to make a salt from a soluble alkali and an acid
In this experiment it is not as easy to determine when all the acidCorrosive substance which has a pH lower than 7. Acidity is caused by a high concentration of hydrogen ions. has been used up as both the acid and alkaliA base which is soluble in water. A base is a substance which neutralises an acid. are colourless solutions.
Therefore, we have to use an indicatorA chemical that gives a colour change in acidic, alkaline or neutral solutions.indicator to determine when neutralisationA reaction between an acid and a base producing a salt and water.neutralisation has taken place.
We can either remove the indicator by using decolourising charcoal, or, once we know the volumes required for neutralisation, we can repeat the experiment using the exact volumes without an indicator.
In this experiment you will prepare dry, pure sodium chloride using hydrochloric acid and sodium hydroxide solution:
hydrochloric acid + sodium hydroxide → sodium chloride + water
WATCH: How to make a salt from a soluble alkali and an acid
Experiment: Making a soluble salt from an alkali
What apparatus and chemicals are used in this experiment?

What are the steps involved in carrying out experiment 2?
Fill the burette with hydrochloric acid.
Using the pipette and pipette filler, place 25cm3 of sodium hydroxide solution into a conical flask.
Add 2 drops of phenolphthalein indicator to the conical flask (it should go pink).
Carefully add the hydrochloric acid from the burette into the conical flask, swirl gently and rinse any droplets down into the flask with deionised water.
Stop adding the hydrochloric acid when the indicator turns colourless and record the volume added by carefully reading the burette.
Repeat steps 1-5 with fresh chemicals, but this time without the indicator, adding the recorded volume of hydrochloric acid.
Carefully pour the solution into an evaporating basin.
Place onto a tripod and gauze and heat gently using a Bunsen burner until about ½ of the solution remains.
When the crystals have formed, filter if necessary and dry (between two sheets of filter paper or using a desiccator or place in a low temperature oven).
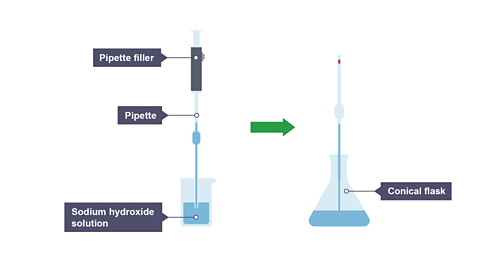
Image caption, 1. 25 cm³ of sodium hydroxide solution is transferred to a conical flask using a pipette.

Image caption, 2. Phenolphthalein solution is added to the conical flask, turning the solution pink.

Image caption, 3. Hydrochloric acid is added until the colour of the phenolphthalein indicator changes from pink to colourless.
1 of 3
What is an alternative method for experiment 2?
Alternative method, using charcoal to remove indicator colour.
Follow steps 1-5:
After step 5, add 2 spatulas of decolourising charcoal to the conical flask.
Heat gently, using tripod, gauze, Bunsen burner and heatproof mat – do not boil.
Allow to cool slightly, then filter the mixture into an evaporating basin.
Place onto a tripod and gauze and heat gently using a Bunsen burner until about ½ of the solution.
When the crystals have formed, filter if necessary and dry (between two sheets of filter paper or using a desiccator or place in a low temperature oven).
How much do you know about prescribed practical C3?
More on Unit 3: Prescribed practicals
Find out more by working through a topic
- count4 of 9

- count5 of 9

- count6 of 9
